Tag: generic manufacturing
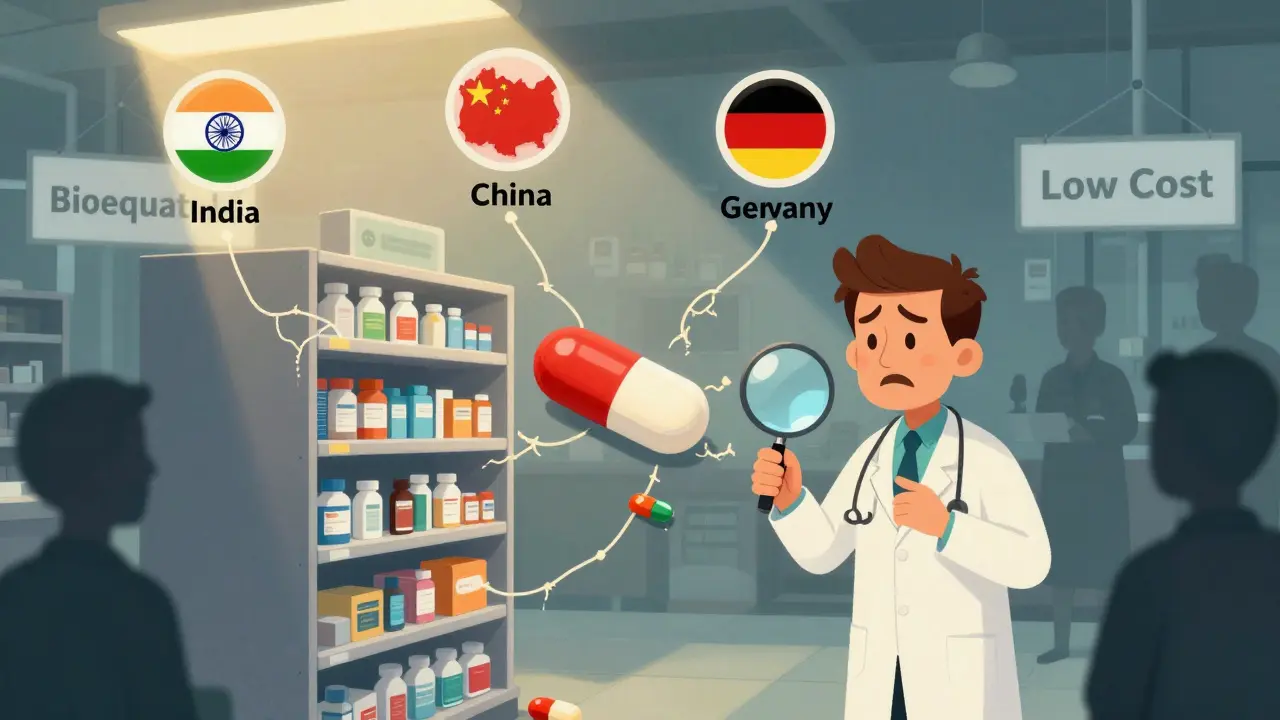
Quality concerns: when clinicians question generic manufacturing
Clinicians are raising alarms about generic drug quality as manufacturing shifts overseas. Studies show higher adverse event rates for drugs made in India, and outdated supply chains are causing dangerous shortages. Transparency and domestic production may be the key to safer generics.
read morerecent posts

SGLT2 inhibitors lower blood sugar but also cause fluid loss, leading to dehydration, dizziness, and blood pressure drops. Learn who's at risk and how to stay safe while taking these powerful diabetes drugs.
Published ON: 11 Feb
Learn the real symptoms, causes, and proven ways to manage type 2 diabetes - from insulin resistance to lifestyle changes and new medications. Understand what works, what doesn't, and how to take control.
Published ON: 7 Feb
The FDA now conducts unannounced inspections at foreign food facilities to ensure imported products meet U.S. safety standards. This shift closes a long-standing gap in oversight and forces overseas plants to maintain compliance 24/7.
Published ON: 3 Feb
Learn the key differences between ACL and meniscus injuries, when surgery is truly needed, recovery timelines, and how to avoid long-term knee problems like arthritis. Real data and patient experiences guide your decisions.
Published ON: 2 Feb
Understanding how medicines work at the molecular level helps you use them safely. From receptor interactions to drug-food conflicts, knowing the mechanism reduces risks and empowers better decisions.
Published ON: 18 Feb