Tag: FDA patient reporting

How to File a Direct Complaint with the FDA as a Patient
Learn how to file a direct complaint with the FDA about adverse reactions or product problems. Step-by-step guide for patients, caregivers, and consumers on using MedWatch, the Safety Reporting Portal, and what happens after you report.
read morerecent posts

China produces 80% of the world's active pharmaceutical ingredients, offering low-cost generics but raising serious quality concerns. FDA inspections reveal widespread lab failures, data fraud, and inconsistent purity - putting patient safety at risk.
Published ON: 25 Feb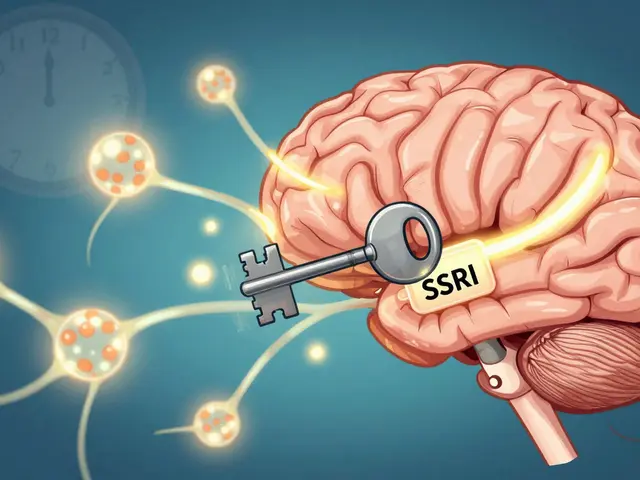
SSRIs are the most common antidepressants used today. Learn how they work in the brain, what side effects to expect, why they take weeks to work, and what to do if they don’t help.
Published ON: 7 Feb
SGLT2 inhibitors lower blood sugar but also cause fluid loss, leading to dehydration, dizziness, and blood pressure drops. Learn who's at risk and how to stay safe while taking these powerful diabetes drugs.
Published ON: 11 Feb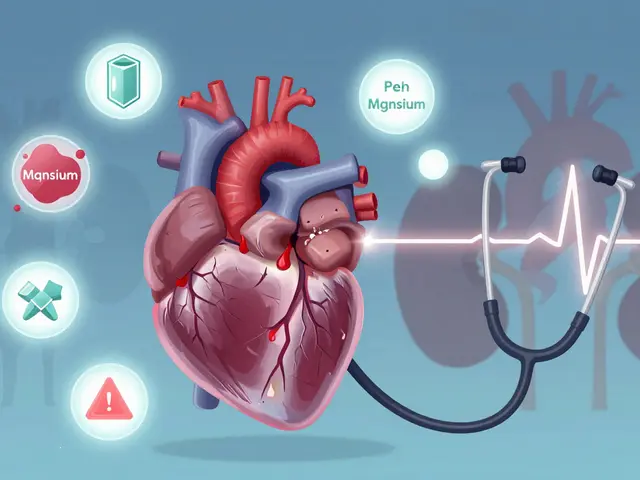
Electrolyte imbalances in potassium, phosphate, and magnesium can cause life-threatening heart and breathing problems. Learn how to recognize the signs, follow life-saving treatment protocols, and prevent complications-especially in kidney disease and critical care.
Published ON: 8 Feb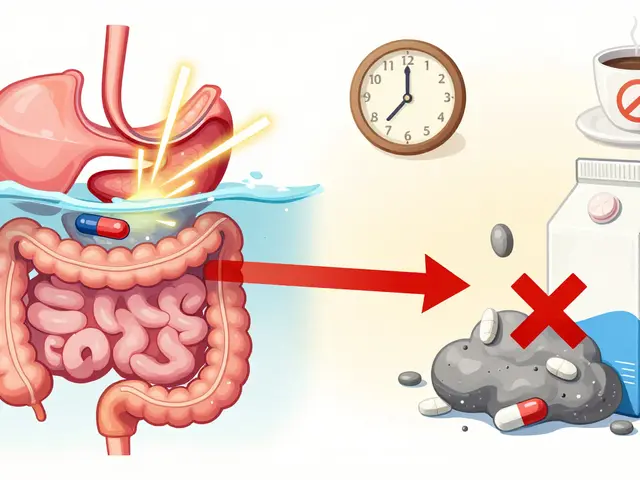
Calcium supplements can block bisphosphonate absorption by up to 90% if taken together. Learn how to time your doses correctly to protect your bones and avoid treatment failure.
Published ON: 21 Feb