
Every year, thousands of people in the U.S. experience unexpected side effects from medications, medical devices, or even dietary supplements. Some of these reactions are serious - maybe even life-changing. But here’s the thing: most of them never get reported. If you’ve had a bad reaction to a drug, a device that failed during use, or a product that didn’t work as promised, you’re not alone. And you can help protect others by filing a direct report with the FDA.
What Exactly Can You Report to the FDA?
You don’t need to be a doctor or a pharmacist to report. The FDA’s MedWatch program lets anyone - patients, family members, caregivers - report problems with FDA-regulated products. That includes:- Drugs (prescription and over-the-counter)
- Biologics (like vaccines or blood products)
- Medical devices (pacemakers, insulin pumps, hearing aids, etc.)
- Dietary supplements
- Infant formula
- Cosmetics
You should report if you’ve experienced:
- A serious side effect (like liver damage, severe allergic reaction, heart rhythm issues)
- A device that broke, malfunctioned, or didn’t work as intended
- A product that didn’t work at all - for example, a birth control pill that failed, or an asthma inhaler that stopped working
- A product with contamination, wrong dosage, or labeling errors
It doesn’t matter if you’re not 100% sure it was the product. If something felt wrong, and it happened after using the product, report it. The FDA’s job is to sort out the patterns - one report might seem small, but 50 similar reports? That’s a red flag.
How to File a Report: Step by Step
There are four ways to file a report. Pick the one that works best for you.- Online via the Safety Reporting Portal (SRP) - This is the fastest and most efficient method. Go to fda.gov/medwatch and click on the portal link. You’ll fill out a simple form asking for your contact info, product details, and a description of what happened. The form takes about 15-20 minutes. But be warned: since August 2024, the portal has had frequent crashes. If it freezes, try again later or use another method.
- Use the FDA 3500 form (paper or PDF) - Download the free FDA Form 3500 from the MedWatch site. Print it, fill it out by hand or on your computer, then mail or fax it. The form is designed to be easy to use - no medical jargon required. You can even get a Spanish version (Form 3500B) if needed.
- Call the FDA - If you’re uncomfortable writing or typing, call 1-800-FDA-1088. A representative will take your report over the phone. Hours are Monday-Friday, 8 a.m. to 8 p.m. Eastern Time. They’ve improved call response times - 92% of calls are answered during business hours.
- Ask your doctor or pharmacist - They can file a report for you, but they might not. Studies show that doctors often skip patient reporting because they’re overwhelmed. If you want your report to go in, do it yourself. Your experience matters more than theirs.
What Information Do You Need?
You don’t need to be a medical expert, but having these details handy will make your report stronger:- Product name - The brand and generic name (e.g., "Lipitor" and "atorvastatin").
- Lot number and expiration date - Look on the packaging. If it’s not there (and it often isn’t), write "unknown." The FDA says 62% of consumer products don’t show lot numbers clearly.
- Date you started using it - When did you begin taking the drug or using the device?
- When the problem started - Did it happen after one dose? After six months?
- What happened - Describe symptoms in plain language: "I had chest pain after taking the pill," not "I experienced acute myocardial ischemia."
- What you did - Did you stop using it? Did you go to the ER? Did you get treatment?
- Other meds or supplements you take - Even OTC stuff like ibuprofen or fish oil.
- Your contact info - They won’t share it. FDA law (21 CFR 10.75) protects your identity.
Don’t worry if you don’t have all the details. Just give what you know. The FDA can follow up if they need more.

Why Your Report Matters
Clinical trials test drugs on a few thousand people. Real life? Millions use the product. Side effects that show up in 1 out of 10,000 users? Those rarely show up in trials. That’s where patient reports come in.In 2024, the FDA took action on 17 safety issues mostly because of patient reports. One example: 287 patients reported insulin pens leaking or delivering wrong doses. That led to a safety warning about improper storage. Another: patients reported a new migraine drug causing sudden vision loss. That triggered a review and new labeling.
And here’s something surprising: patient reports often include more detail about daily life than doctor reports. A 2023 study found patient reports had 37% more information about when symptoms started and 28% more info about over-the-counter drugs they were using. That’s because you know your body - not your doctor’s notes.
What Doesn’t Work
The system isn’t perfect. Here’s what to expect:- Technical problems - The online portal has been glitchy since August 2024. If it crashes, don’t give up. Use the phone or paper form.
- Underreporting - Studies estimate 90-95% of adverse events go unreported. That means the FDA only sees a tiny slice of the problem.
- Language barrier - Only English and Spanish forms are available. If you speak another language, you’ll need help filling it out.
- No confirmation - Only 34% of people know they get a confirmation email within 5 days. Check your spam folder.
- Slow processing - Even with new AI tools, it takes 9-15 business days for your report to show up in the system. That’s better than the old 22-day wait, but still not fast enough for urgent cases.
Don’t let these issues stop you. The FDA needs every report. Your voice fills the gaps.
What Happens After You Submit?
Your report goes into the FDA Adverse Event Reporting System (FAERS), which holds over 25 million reports since 1969. Trained analysts look for patterns. If 10 or more people report the same issue with the same product, it gets flagged. If the signal is strong enough, the FDA might:- Update the product label with new warnings
- Require the manufacturer to run a new safety study
- Issue a public safety alert
- Request a product recall
You won’t get updates unless you ask. But your report could save someone else’s life - maybe even your own next time you use the product.
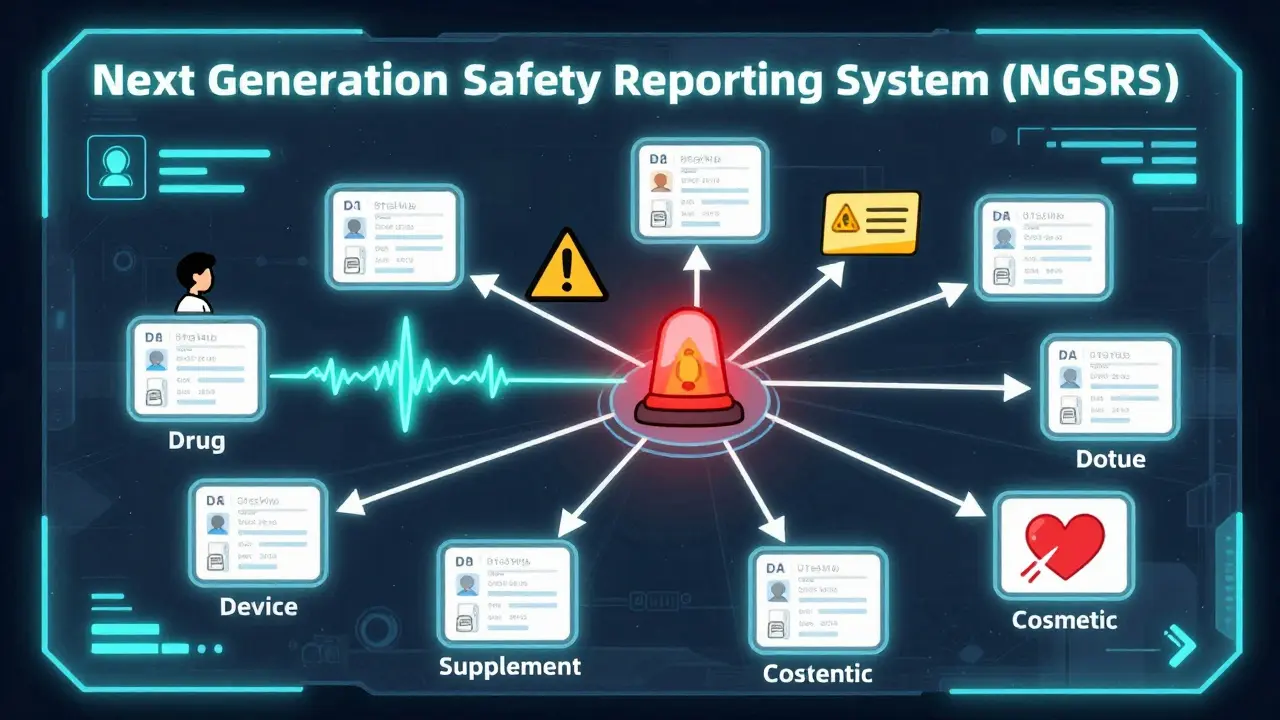
What’s Changing in 2025 and Beyond
The FDA is upgrading the system. In January 2025, they launched the Next Generation Safety Reporting System (NGSRS). It now:- Automatically checks lot numbers against manufacturer databases
- Uses AI to suggest symptom codes so you don’t have to guess medical terms
- Has a new symptom wizard built into the online form - it reduced terminology errors by 33% in testing
By 2026, they plan to cut processing time to just 5 business days. By 2027, they’ll add reporting in five more languages. And by 2028, they aim to merge all six separate reporting portals into one - no more confusion between drug, device, or supplement forms.
These changes are happening because patients like you spoke up.
Real Stories Behind the Numbers
On Reddit, one user named MedWatcher2024 said they tried to report a severe reaction to a new migraine drug in March 2025 - the portal crashed three times. They ended up mailing the paper form. It took weeks, but it got through. Another patient, living with cancer, said the Spanish version of the form (3500B) helped her document her immunotherapy reaction when her oncologist didn’t have time to file it. "It was the only way I could make sure they knew what happened," she wrote. These aren’t outliers. They’re proof that the system works - if you use it.What to Do Next
If you’ve had a bad experience:- Don’t wait. File your report now.
- Use the paper form if the website is down.
- Call 1-800-FDA-1088 if you’re stuck.
- Keep a copy of your report for your records.
- Tell others. Many people don’t even know this option exists.
You don’t need permission. You don’t need proof. You just need to speak up. Your report could be the one that triggers a warning, a recall, or a life-saving change. The FDA can’t fix what it doesn’t know. And right now, it’s only hearing a fraction of what’s out there.
Can I report a problem even if I’m not sure it was caused by the product?
Yes. The FDA understands that it’s often hard to know for sure what caused a reaction. Your report helps them spot patterns. If multiple people report similar issues with the same product, they can investigate further. Even if you’re unsure, report it.
Will my name be made public if I report something?
No. By law (21 CFR 10.75), the FDA cannot disclose your identity without your written consent. Your personal information is protected and kept confidential. Only the FDA and authorized reviewers see your details.
Do I need a doctor’s note to file a report?
No. You don’t need medical records, prescriptions, or a doctor’s signature. The FDA accepts reports based on your personal experience. However, including details like when symptoms started, how long they lasted, and what you did to treat them helps them understand your case better.
How long does it take for the FDA to act on a report?
It varies. Most reports are reviewed within 9-15 business days. But action - like a label change or recall - can take months or even years, depending on how many similar reports come in and how strong the evidence is. Your report is part of a larger picture. It may take time, but it matters.
Can I report a problem with a supplement or cosmetic?
Yes. The FDA regulates dietary supplements and cosmetics, and you can report adverse events with these products through MedWatch. While the FDA has less authority over supplements than drugs, patient reports help identify dangerous ingredients or misleading claims that might lead to future action.




12 Comments
The FDA's entire reporting system is a glorified dumpster fire wrapped in a compliance blanket. You think they care about your 'personal experience'? Nah. They're drowning in 25 million reports and their AI 'symptom wizard' just auto-tags everything as 'headache' or 'nausea'. I filed a report about my pacemaker glitching during a workout - they replied with a form letter that said 'your case has been reviewed' and then archived it. Meanwhile, the same device killed three people in Ohio and nobody even blinked. This isn't patient advocacy - it's bureaucratic theater.
Why do we even bother reporting to the FDA when the real power lies with the manufacturers who fund their research and write the labels in the first place? You think your report changes anything? It just feeds the machine that lets them keep selling dangerous shit under the guise of 'clinical trials' and 'risk-benefit analysis'. The system is rigged. They don't want to know what's really happening - they want to pretend they're doing something while the profits keep rolling in.
Did you know that the FDA’s MedWatch portal was originally designed by a contractor who later got hired by Pfizer? And that the same contractor also helped design the algorithm that filters out 'duplicate' reports - which means if 50 people report the same side effect but use slightly different wording, the system deletes 47 of them as 'duplicates'? This isn't incompetence - it's intentional suppression. The government and Big Pharma are in bed together and your report? It’s just noise they’re trained to ignore. I’ve got screenshots of my 2021 report that got auto-deleted. They don’t want you to know this. But now you do.
It's good that people are finally talking about this. I filed a report last year after my insulin pump stopped working during a night hike - I was lucky I had a backup. The online form crashed twice, so I called 1-800-FDA-1088. The rep was calm, listened, and took notes. Took three weeks but I got a confirmation email. It felt like a small win. Not everyone has access to this info. Maybe we need more community workshops - libraries, churches, clinics - teaching people how to report without needing a medical degree.
It is of the utmost importance that we recognize the profound societal responsibility that each individual holds when submitting an adverse event report to the Federal Drug Administration. The sanctity of public health is predicated upon the conscientious and meticulous documentation of personal experiences - not merely as anecdotes, but as data points within a vast, intricate, and morally imperative epistemological framework. Your voice, however seemingly inconsequential, is a vital thread in the tapestry of patient sovereignty. Do not underestimate the cumulative weight of your testimony. It is not merely a form - it is an act of civic courage. Submit. Document. Persist.
Man, I remember when I tried to report my daughter’s allergic reaction to a new OTC cough syrup. The website crashed so I printed the 3500 form, filled it out with a Sharpie, and mailed it certified mail. Two months later, I got a letter saying they'd added a warning label. Not because of me - but because I was one of 87 people who reported the same thing. You don’t need to be loud. You just need to be consistent. One report won’t change the world. But 10,000? That’s a revolution.
It is disgraceful that the United States allows its citizens to report adverse events in a system that is so inefficient and unregulated. In India, we have a centralized, state-mandated reporting mechanism that is monitored by trained pharmacovigilance officers who respond within 72 hours. Why does America rely on citizens to do the government’s job? This is not freedom - this is negligence. You should be ashamed to live in a country where your life depends on whether you have time to fill out a PDF form.
Hey I just filed my report last week after my birth control pill failed - didn't even know I could do it myself! I used the paper form because the website kept timing out. Took me like 20 mins. They didn't ask for anything crazy, just the name of the pill and when I started feeling weird. I felt good about it. I told my mom and she's gonna file hers too. It's kinda empowering, honestly. Like, you're not just a patient - you're part of the fix.
Look, I used to think reporting was pointless - until my best friend had a seizure after taking a new migraine drug. We filed the report together. Three months later, the FDA added a black box warning. She’s alive because of it. You think you’re just typing into a form? You’re saving someone’s future self. Don’t wait for someone else to do it. Do it now. Seriously. Go. I’ll wait.
I love that the FDA’s new AI wizard suggests symptom codes - I had no idea how to describe my tingling hands after the supplement. I typed 'my fingers feel like they're buzzing' and it suggested 'paresthesia'. I didn’t even know that word! But I kept my original description too. It felt good to be heard, not just categorized. Also, the Spanish form saved my abuela’s life - she couldn’t read English but she could fill out 3500B with help from her grandson. That’s inclusion. That’s care.
I appreciate the clarity of this guide. One small correction: the FDA does not guarantee confidentiality under 21 CFR 10.75 if the report contains information that could be used to identify you - such as a unique combination of age, location, and condition. While they protect your name, they may still be required to disclose non-identifying details in response to FOIA requests. Always assume your data could become part of a public dataset. Still worth reporting - just be informed.