
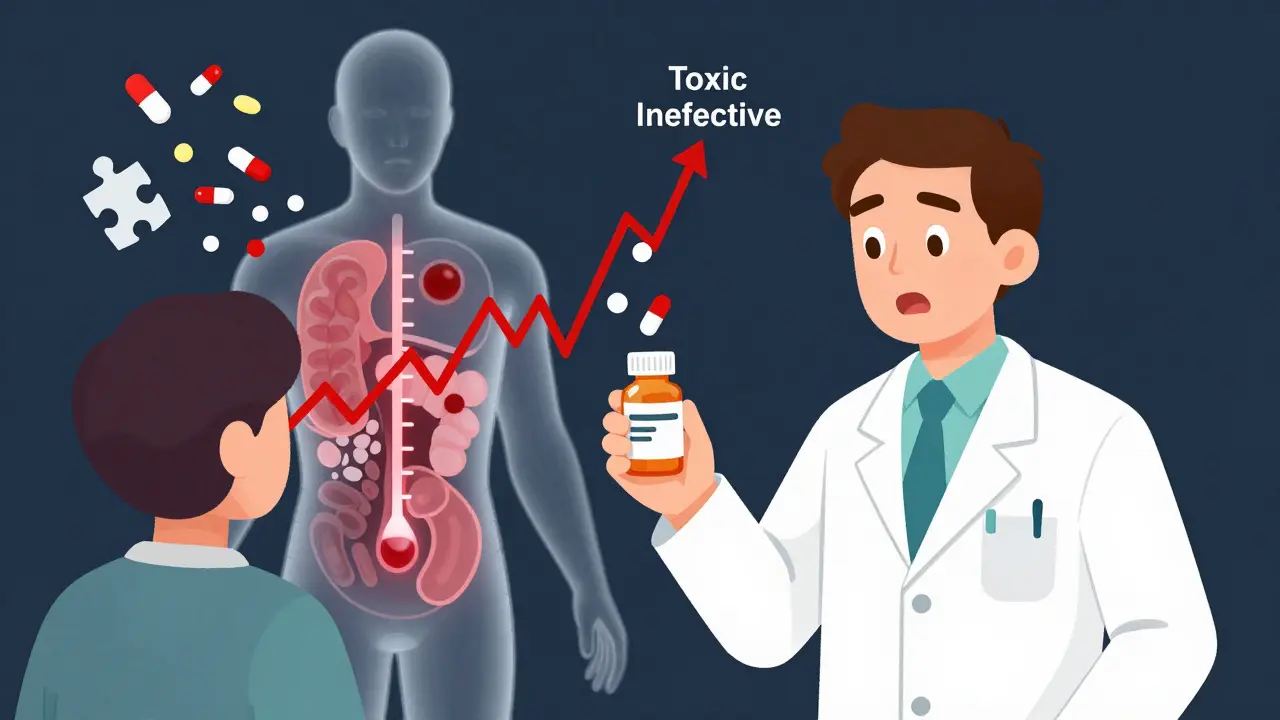
When you take a medication like levothyroxine or tacrolimus, even a tiny change in dose can mean the difference between feeling fine and ending up in the hospital. These are called Narrow Therapeutic Index (NTI) drugs - medicines where the line between safe and dangerous is razor-thin. A 5% increase in blood concentration might cause toxicity. A 5% drop might make the drug useless. And when you switch from one generic version to another - even if both are labeled the same - that small shift can happen without you knowing.
What Makes a Drug an NTI Drug?
NTI drugs have a very small window between the dose that works and the dose that harms. The FDA doesn’t publish a full list, but it’s clear which ones matter: warfarin, digoxin, lithium, phenytoin, carbamazepine, cyclosporine, and tacrolimus. These aren’t just any pills. They’re the kind you take for life - for thyroid function, seizures, organ transplants, or heart rhythm control.
The therapeutic index tells the story. For lithium, it’s about 2:1. That means the toxic dose is only twice the effective dose. For digoxin, it’s the same. One pill from one manufacturer might give you 100% of the active ingredient. Another might give you 93%. That’s within FDA’s bioequivalence rules - but for an NTI drug, that 7% difference can throw off your entire treatment.
How Are Generic NTI Drugs Approved?
The FDA says generic NTI drugs are just as good as the brand. But here’s the catch: they require stricter testing. For most drugs, generics must match the brand within 80% to 125% of the active ingredient’s absorption. For NTI drugs? That range is tighter - often 90% to 111%, sometimes even 95% to 105%. That’s not just a tweak. It’s a major upgrade in quality control.
Still, even with tighter limits, real-world results vary. A 2019 study looked at four different generic versions of tacrolimus. One had 93% of the active ingredient. Another had 110%. The brand was 100%. All passed FDA standards. But in transplant patients, those differences showed up in blood levels. One patient’s tacrolimus level jumped 30% after switching from one generic to another - and they had a rejection episode.
Switching Generics: What Happens in Real Life?
Pharmacists are trained to substitute generics. It’s standard practice. But for NTI drugs, it’s not always safe. A 2019 survey of 710 pharmacists found that 87% believed generics were just as effective. But 63% said they’d gotten complaints from doctors or patients after switching between generic brands.
Take levothyroxine. A 2021 FDA analysis of over 10,000 patients found no difference in TSH levels between brand-name Synthroid and generic versions. Mean TSH was 2.12 vs. 2.15 - statistically the same. So why do some patients report fatigue, weight gain, or heart palpitations after switching?
Because biology isn’t a spreadsheet. One person’s body absorbs a pill differently than another’s. If you’ve been stable on one generic for years, then your pharmacist switches you to a different one because of cost - your thyroid levels might drift. You might not notice until your next blood test. And by then, your TSH could be 4.5 instead of 2.0. That’s not a big number on a lab sheet. But it’s enough to make you feel awful for months.
Why Do Some Doctors Resist Switching?
Neurologists are especially cautious with drugs like phenytoin and carbamazepine. The American Academy of Neurology doesn’t recommend automatic substitution of antiepileptic generics - even when the FDA says they’re interchangeable. Why? Because seizures don’t care about bioequivalence studies. A patient might be seizure-free for five years on one generic. Switch to another, and they have a breakthrough seizure. No one can predict who that patient will be.
A 2015 study of kidney transplant patients showed a 15.3% higher rate of acute rejection when switching from one cyclosporine formulation to another. That’s not a fluke. That’s a clinical red flag. And it’s not just about the active ingredient. The fillers, coatings, and manufacturing processes can change how the drug dissolves in your gut. For an NTI drug, that’s enough to matter.
State Laws Are Trying to Catch Up
As of 2022, 27 U.S. states have laws that restrict or require special permission before substituting NTI drugs. In those states, pharmacists can’t swap generics for drugs like warfarin or lithium without the prescriber’s okay. Some require the pharmacist to notify the doctor. Others require the patient to sign a form.
But in the other 23 states? It’s still automatic. You walk in for your refill, and the pharmacist hands you a different bottle. You don’t know it’s a different manufacturer. You don’t know to ask. And if you feel off? You might blame stress, diet, or aging - not the pill.

What You Can Do to Stay Safe
If you take an NTI drug, here’s what you need to do:
- Know your drug. Is it on the FDA’s list of NTI drugs? If you’re on warfarin, lithium, tacrolimus, or levothyroxine, assume it is.
- Check the label. Every time you get a refill, look at the manufacturer name. If it changes, ask your pharmacist why.
- Ask for a hold. Tell your doctor and pharmacist: “I don’t want to switch manufacturers without talking to you first.” Many will honor that request.
- Track your numbers. If you’re on warfarin, monitor your INR closely after any switch. If you’re on levothyroxine, get your TSH checked 6-8 weeks after switching. If you’re on tacrolimus, ask for a blood level test.
- Speak up. If you feel different - tired, dizzy, anxious, or unwell - after a switch, say so. Don’t wait. Bring your pill bottle to your next appointment.
Is There a Better Way?
The FDA insists generic NTI drugs are safe. And for most people, they are. Real-world data shows no major spike in hospitalizations or deaths from switching. But safety isn’t just about population averages. It’s about individual risk.
Some experts argue that NTI drugs should be treated like insulin or chemotherapy - no automatic substitution. Others say that’s unrealistic. Generics save billions. Patients need access. The solution might be balance: stricter testing, better labeling, and patient-centered choices.
Right now, the system is built for efficiency - not for the person who’s one pill away from disaster. Until we fix that, the safest thing you can do is stay informed. Your life might depend on it.
Are all generic drugs the same, even for NTI medications?
No. While all generics must meet FDA bioequivalence standards, NTI drugs have tighter limits - and even within those limits, small differences in absorption can occur between manufacturers. Two generics labeled the same may have different fillers, coatings, or release profiles, which can affect how your body absorbs the drug. For drugs like tacrolimus or warfarin, those differences can be clinically meaningful.
Can I switch between brand-name and generic NTI drugs safely?
For many patients, yes. The FDA approves these switches based on rigorous testing. Real-world studies on levothyroxine show no significant change in TSH levels when switching between brand and generic. But if you’ve been stable on one version - whether brand or generic - changing to another increases your risk of fluctuation. Talk to your doctor before switching, especially if you’ve had issues before.
Why do pharmacists switch NTI generics without telling me?
In most states, pharmacists are legally allowed to substitute generics without notifying the patient or prescriber. It’s done to save money and streamline filling. But for NTI drugs, this practice is controversial. Some states require disclosure or consent. If you’re concerned, ask your pharmacist to note your preference on file: “No substitutions for NTI drugs.”
What should I do if I think a generic switch caused side effects?
First, don’t stop your medication. Contact your doctor immediately. Bring the new pill bottle with you. Your doctor can order a blood test - like an INR for warfarin or a trough level for tacrolimus - to see if your levels changed. If they did, they may switch you back or request a specific manufacturer. Document the timing: when you switched, when symptoms started. That helps pinpoint the cause.
Is there a list of NTI drugs I can check?
The FDA doesn’t publish a complete official list, but it has identified key NTI drugs in guidance documents. These include warfarin, levothyroxine, lithium, phenytoin, carbamazepine, digoxin, cyclosporine, tacrolimus, and theophylline. If you’re unsure whether your drug is an NTI drug, ask your pharmacist or check the FDA’s product-specific bioequivalence guidance documents.




15 Comments
This is wild - in India, we get generics so cheap you’d think they’re candy… but then you hear stories of people on tacrolimus crashing after a switch and it hits different. I’ve seen a cousin go from stable to ICU over a pill change. No one warned us. No one asked. Just… swapped. The system’s broken.
The FDA is complicit. This isn’t about bioequivalence - it’s about corporate lobbying. The same conglomerates that own brand-name drugs also own the generics. They’re testing on populations with different metabolisms, then selling the same pill under different labels. You think this is about safety? It’s about profit margins disguised as science.
I’ve been on levothyroxine for 12 years. Switched generics twice - once felt like I was dragging through wet cement for months. TSH was normal on paper, but my body knew. I now insist on the same brand. My doctor respects it. It’s not about being difficult - it’s about listening to your own body when the numbers lie.
Let’s be real - 90% of people who say they ‘feel different’ after a switch are just anxious. The FDA’s data is rock solid. If you’re having symptoms, it’s more likely your stress, diet, or sleep. Stop blaming the pill. Also, why do people think their personal experience overrides clinical trials? It’s not magic. It’s pharmacokinetics.
Oh, the tragic irony of the American pharmaceutical hellscape - where the very molecules meant to sustain life are reduced to interchangeable commodities, stripped of nuance, divorced from the sacred intimacy of biological individuality. We commodify the human body’s delicate equilibrium, then wonder why people feel like ghosts in their own skin. The system doesn’t fail - it was designed this way.
It’s disgraceful that American citizens are treated like lab rats in a profit-driven experiment. Other developed nations regulate NTI drug substitutions with strict protocols. We don’t. We let pharmacists gamble with people’s lives because ‘efficiency’ sounds better than ‘ethical responsibility.’ This isn’t capitalism - it’s medical negligence dressed in corporate jargon.
Big Pharma doesn’t want you to know this - but the real reason generics vary is because the FDA lets them use different fillers that alter absorption. Some contain gluten. Some have dyes that trigger immune responses. And the worst part? They don’t have to list it. They just slap ‘bioequivalent’ on the bottle and call it a day. You’re being poisoned by invisible ingredients.
If you’re on an NTI drug, don’t wait for the system to fix itself. Take control. Ask for a prescription that says ‘Dispense as written’ or ‘Do not substitute.’ Most prescribers will sign it. It’s your right. Your health isn’t a cost-saving metric. Stand up. Speak up. You’re not being difficult - you’re being smart
My mom’s on warfarin and switched generics last year - ended up with a blood clot. She didn’t even realize it was the pill until her doctor pulled up the records. Now we keep a log: brand, batch, date. We even take pictures of the bottle. It’s annoying but worth it. If you’re on one of these meds, be the weird one who asks questions. You’ll thank yourself later.
I used to think generics were all the same until I switched from one levothyroxine to another and went from feeling great to crying in the shower every day for three months. My doctor said my TSH was fine, but I knew something was off. I went back to the original brand and boom - energy returned. Turns out my body doesn’t care about FDA averages. It cares about what works for ME. Don’t ignore your gut.
It is of paramount importance to recognize that the regulatory frameworks governing bioequivalence for narrow therapeutic index pharmaceuticals are inherently inadequate in addressing interindividual pharmacokinetic variability. The current paradigm prioritizes population-level statistical equivalence over individualized therapeutic outcomes, thereby constituting a systemic failure in patient-centered pharmacotherapy. Regulatory reform is not merely advisable - it is ethically imperative.
My uncle’s a transplant patient - switched generics once, got rejected. Now he only takes the brand. His insurance hates it but he won’t budge. We all thought generics were the same until we saw what happened. Don’t risk your life for $5. Talk to your doc. Get it in writing. You’re not being high maintenance - you’re being alive.
They’re hiding something. I’ve read the documents. The same companies that make the brand also own the generics. The FDA inspectors are paid by them. The ‘tighter’ standards? A PR stunt. You think they’d let a 7% variation fly if it was insulin? No. But for thyroid pills? Eh. We’re disposable. This is controlled chaos.
Back home in Punjab, we don’t even have access to branded meds - generics are all we get. But here’s the thing - we’ve been taking them for decades without issues. Maybe it’s not the pill. Maybe it’s how we track it. In the US, people overthink everything. If your doctor says it’s fine, trust them. Not every switch is a disaster. Don’t scare people with horror stories.
One sentence: If you’re on an NTI drug, never accept a substitution without asking. Write it on your prescription. Keep a pill log. Test your levels. You are your own best advocate. No one else is watching out for you - so do it yourself.