
What the FDA Actually Does at Foreign Food Plants
The U.S. Food and Drug Administration doesn’t just check labels or review paperwork when it shows up at a food factory overseas. It walks through the facility, watches how workers handle ingredients, checks refrigeration temperatures, reviews sanitation logs, and even takes photos of equipment. These aren’t guided tours. Since May 2024, the FDA has been conducting unannounced inspections at foreign food facilities - the same way it does in the U.S. No heads-up. No time to clean up. No last-minute fixes.
This shift wasn’t made lightly. Before 2024, foreign plants got weeks of notice before an FDA inspector arrived. That gave them time to hire translators, polish floors, and hide issues. Meanwhile, American factories were being checked without warning - because the FDA assumed they were always supposed to be clean. That double standard is gone. Now, every facility, no matter where it’s located, must be ready at all times.
Why This Matters for Your Grocery Cart
One in every seven bites of food you eat in the U.S. comes from overseas. That’s 15% of the entire food supply, according to USDA data from 2023. It includes spices from India, seafood from Vietnam, canned tomatoes from Italy, and baby formula from Germany. If a single facility overseas cuts corners - say, by using dirty water to rinse produce or storing raw meat next to ready-to-eat meals - the contamination can spread across the country before anyone notices.
The FDA’s job is to catch those problems before they reach store shelves. In 2023, nearly 300,000 foreign facilities were registered to export food to the U.S. That’s more than the total number of U.S. food plants. With only about 200 inspectors assigned to international inspections, the FDA can’t visit them all. So it picks the riskiest ones.
How the FDA Picks Which Factories to Visit
Not every foreign plant gets inspected every year. The FDA uses a risk-based system to decide who gets visited and when. Three things matter most:
- What’s being made - Raw meat, sprouts, and shellfish carry higher risk than canned beans or bottled water.
- How it’s made - Facilities that do complex processing, like adding preservatives or pasteurizing milk, are more likely to be flagged than those that just package pre-made goods.
- History of trouble - If a plant’s products have been refused entry into the U.S. before, it jumps to the top of the list. Refusals happen when inspectors find unsanitary conditions, undeclared allergens, or illegal chemicals.
Under the FDA Food Safety Modernization Act (FSMA), passed in 2011, the agency was required to double foreign inspections every year for five years. That push helped reverse a sharp drop in inspections during the Trump administration, when funding cuts led to a historic low in overseas checks, according to ProPublica’s 2018 report.

What Happens If a Factory Says No
You can’t say no to an FDA inspector. Not anymore. Section 306 of FSMA gives the FDA the power to block any food from entering the U.S. if the foreign facility refuses inspection. That’s not a warning. It’s a hard stop. The product gets turned away at the border. The company gets added to a public list of “refused” importers. Repeat offenders face longer delays and higher scrutiny.
But refusal isn’t just about locking the door. The FDA considers any action that blocks access to be a violation. That includes:
- Delaying inspectors for hours while they wait for a manager to arrive
- Turning off cameras or shutting down production during the visit
- Redacting parts of records or refusing to show digital files
- Limiting where inspectors can go - like blocking them from the packing line
These aren’t just administrative offenses. The U.S. Department of Justice can pursue criminal charges. Fines can run into millions of dollars. Companies can be barred from exporting to the U.S. for years. The FDA doesn’t just want compliance - it wants proof that compliance is built into daily operations, not just during inspections.
How Foreign Factories Are Adapting (or Struggling)
For many small overseas producers, the change has been brutal. In countries where advance notice was standard, factories used to treat FDA visits like scheduled maintenance. Now, they need to be inspection-ready 24/7. That means:
- Hiring full-time bilingual quality control staff - not just translators for inspection day
- Keeping digital records accessible at all times, even on weekends
- Training every employee to answer basic questions about sanitation and traceability
- Running monthly mock inspections to spot gaps before the FDA does
Some companies have hired U.S.-based consultants to help them prepare. Others are investing in cloud-based systems that automatically log temperature readings, cleaning schedules, and employee training. The goal? To make it impossible to fake compliance.
But challenges remain. Language barriers still cause misunderstandings. Cultural differences in how authority is viewed can lead to tense situations. One inspector told a reporter that a facility in India once refused to let her photograph a machine because “it was too sensitive for foreigners to see.” That’s now a violation.
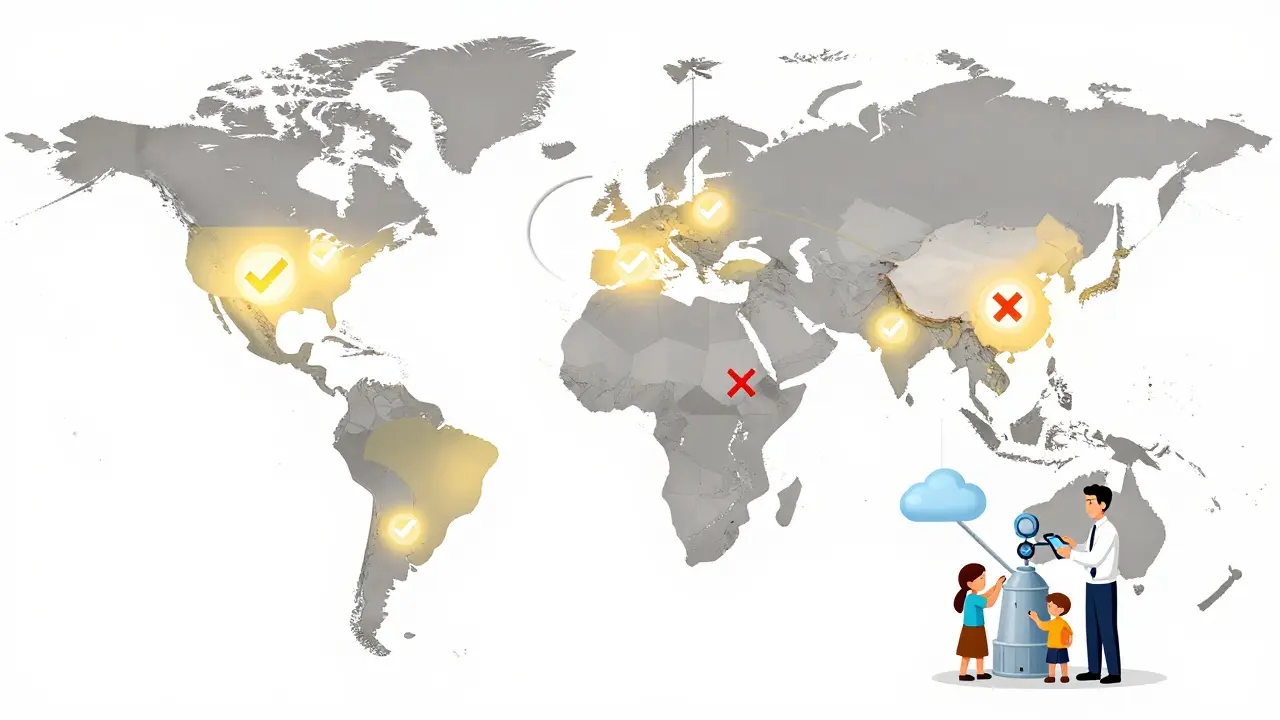
The Bigger Picture: Global Standards Are Rising
The FDA isn’t just enforcing rules - it’s setting a global benchmark. Countries like Japan, Canada, and the EU are watching closely. Some are adopting similar unannounced inspection models. Others are strengthening their own oversight to avoid being seen as “low-risk” and getting targeted by U.S. refusals.
For large multinational food companies, the change is manageable. They already operate under strict global standards. But for small farms and family-run processors, the cost of compliance is steep. Some have stopped exporting to the U.S. altogether. Others are forming cooperatives to share the cost of quality staff and digital systems.
The FDA admits it can’t inspect every facility. But it’s using data smarter now. If a facility’s products keep getting refused, it gets flagged. If a country has a pattern of violations, the FDA increases inspections there. It’s not about punishment - it’s about incentives. The message is clear: if you want to sell food in the U.S., you play by American rules. No exceptions.
What You Can Expect in the Next Few Years
The FDA is already testing new tools. One pilot program in China uses third-party auditors trained and certified by the FDA to conduct initial checks. The agency reviews their reports and follows up with surprise visits. Another project is rolling out AI-powered risk scoring - analyzing past import refusals, supplier histories, and even weather patterns that could affect contamination risk.
Expect more digital requirements. Soon, facilities may need to submit inspection-ready records electronically before an inspector even lands. Paper logs will become rare. Real-time monitoring of cold chain temperatures could become mandatory for high-risk products.
The bottom line? The days of treating U.S. food safety rules as optional are over. Whether you’re a factory in Thailand, a packing house in Mexico, or a spice mill in Morocco - if you want to export to the U.S., you need to operate like you’re in Ohio. Always ready. Always clean. Always honest.



15 Comments
And don’t even get me started on the ‘cultural differences’ excuse. Dirty water isn’t a tradition, it’s negligence.
And honestly? If you’re a small producer in India or Vietnam and you’re struggling? Get help. There are nonprofit groups that offer free compliance training. You don’t have to go it alone. 💪
Moreover, the notion that 'unannounced inspections' equate to fairness is laughable. What about due process? What about jurisdictional sovereignty?
And let's not forget: 98% of refusals are for minor documentation errors - not botulism. The real agenda? Market protectionism dressed up as public health.
Who elected them? Who authorized them to dictate sanitation protocols in Morocco, Thailand, or Mexico?
This is not food safety - this is regulatory colonialism. And the fact that people cheer this as ‘progress’ reveals a disturbing moral bankruptcy in modern American exceptionalism.
They inspect shrimp farms in Vietnam because they’re ‘high-risk,’ but ignore the 300+ tonne soybean importers from Brazil that have been linked to aflatoxin outbreaks since 2019?
And let’s be honest - AI-powered scoring? That’s just algorithmic racism wrapped in a white lab coat. You can’t quantify ‘sanitation’ with a machine trained on U.S. norms.
Also - ‘mock inspections’? That’s not compliance. That’s performance art.
It’s not easy, but it’s doable. Start with one thing: get a free FDA Food Safety Plan template. Do it tonight. You’ve got this. 🙌
Have you ever tried explaining FDA protocols to someone who’s never seen a clipboard before? It’s not just about rules - it’s about trust. And right now, there’s none.
We see you. We’re rooting for you. And you’re not alone in this.
One day, your child will eat a meal made from your work - and it will be safe. That’s worth everything.
And don’t even get me started on the ‘AI risk scoring’ - I bet it flags ‘spices’ as high risk because it thinks ‘spice’ = ‘danger’ like in some 1950s horror movie. LOL.
Also - ‘mock inspections’? More like ‘drama rehearsals’ with a PowerPoint. 🤡
Start with one thing today. Maybe just write down your cleaning schedule. Or take a photo of your fridge temp.
You don’t need to fix everything at once. Just start. I believe in you.
Do we really think a machine can measure trust? Or that a checklist can replace dignity?
The FDA wants compliance - but what if the real solution is connection? What if we trained inspectors to listen as much as they record?
Maybe the goal shouldn’t be ‘zero refusals’… but ‘zero fear.’
Yes, it cost us. But we doubled our U.S. orders last year.
This isn’t about American rules. It’s about respect. And respect pays.